Laboratory investigation of Surfactant and Polymer formulation design for Heavy Oil recovery in sand stone reservoirs
Chemical
flooding is classed as an important technique of Enhanced Oil Recovery (EOR)
which has been applied throughout the globe since the 1980s. The outcomes of
such flooding have mainly been effective in both onshore and offshore locations
with the latter being more challenging due to factors such as weather;
challenges associated with logistics of chemicals and high salinity content in
sea waters. Nonetheless, there has been significant incremental oil recovered
in offshore location especially during the last couple of decades (Manrique
2007). The contributing factors behind the success rate of such flooding are
mainly due to the combination of chemicals used. The surfactant and polymer
used in chemical flooding would help in producing more oil which was previously
out of reach through various mechanisms such as the lowering of Interfacial
Tension (IFT) between the phases in the reservoir as well as improving the
sweep efficiency.
Vast
amount of research has been done on chemical flooding, however, the effect of
various combinations of chemicals used in Alkaline, Surfactant, Polymer (ASP)
flooding on the mechanism behind changes in the capillary pressures have not been
fully clarified (Shen, 2007).
Research carried out by Zhang et al.
(2012) indicated that it was possible was to develop an ASP flood which could
recover incremental oil at low surfactant concentrations. They performed a
series of phase behavior tests, interfacial tension (IFT) measurements and core
flood test in order to develop a solution for oil that had an API gravity of 15
and viscosity of 2,000 cp. The result indicated an incremental recovery of 6 %
in comparison with previous water flooding (Zhang, 2012).
Furthermore,
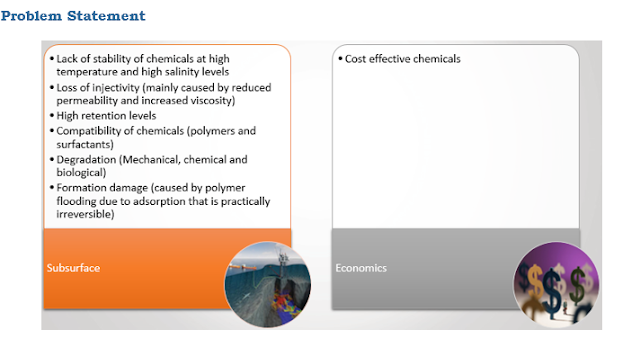
in recent research by Sheng, 2013, the need for development of ASP formulation
with high temperature and high salinity limit has been highlighted. The
advantage of ASP formulation over its individual process is the synergy between
the polymer, surfactant and alkaline used. To have this synergy, the components
of alkaline, surfactant and polymer must be in the same slug (Sheng, 2013). For
example, in alkaline-polymer flooding, alkaline reaction with crude oil results
in soap generation, wettability alteration, and emulsification; and polymer
provides the required mobility control. Alkaline-polymer flooding can displace
more residual oil than individual alkaline flooding or polymer flooding (Sheng,
2013).
Moreover, it was reported by Sheng, 2013
that when surfactant and polymer were injected in the same slug (SP flooding),
their compatibility was an issue. In some cases, polymer was injected before
surfactant as a sacrificial agent for adsorption or for conformance improvement.
Sometimes polymer was injected behind surfactant to avoid chase water fingering
in the surfactant slug. Even though polymer was not injected with surfactant in
the same slug, they mixed at their interface because of dispersion and
diffusion. Polymer may also mix with surfactant owing to the inaccessible pore
volume phenomenon when it is injected behind surfactant. Sheng referred to
these phenomena as surfactant-polymer interaction or incompatibility. Cases
such as this are examples of the possible interaction that polymer and
surfactant can have once injected into the reservoir. These interaction
sometimes have negative or positive effect of the performance of the chemicals.
In other researches, the effectiveness
of associative polymers in displacing of oil has been reported (Wever, 2011).
The main attraction of associative polymers for EOR is their significant
viscosity enhancement ability compared with conventional polymers and their
potential salinity resistance in real oil field applications (Wever, 2011).
An example of an associative polymer is
Phenyl-polyacrylamide (PPAM) which is a hydrophobic synthesized Polyacrylamide
(PAM). Such synthesized polymers can be used as a more effective mobility
control agent in future research. It is worth mentioning that PPAM is a
non-commercial type of polymer which has been developed in laboratory at London
South Bank University. The results of recent tests carried out at LSBU showed
that PPAM had higher resistance to salinity conditions and also higher
viscosity at the same concentration compared to PAM.
Research Aims
The aim of this research is to study the
synergy between the use of hydrophobic modified acrylamide and series of surfactants.
This would be achieved through understanding the synergy between the candidate
surfactants and polymers such as PPAM used in the core flooding. The subsequent
findings around the synergetic effects will be used to design an effective
chemical flooding more resistant to salinity and thermal conditions with the
view of achieving higher efficiency in fluid displacement.
Research Objectives
·
Study
in detail the chemical composition and structure of candidate polymer,
surfactant and alkaline
·
Understand
in details the synergy between the candidate polymer, surfactant and alkaline.
·
Develop
an ASP formulation, with tolerance to high salinity and high temperature
conditions, based on the understanding around the synergy of candidate polymer,
surfactant and alkaline.
Research Methodology
Experimental
work
It is of importance to embrace the
candidate reservoir characteristics in order to design EOR fluid. One way of
accomplishing this is through laboratory analysis where fluid-fluid and
rock-fluid interactions are evaluated. Doing so, it is possible to introduce
solutions tailored to each candidate reservoir.
Series of laboratory experiments are listed below:
·
Injectivity and sweep efficiency (core flooding)
·
Filterability test: To evaluate the presence of undissolved solids in the
SP solution and to identify a possible plugging behaviour.
·
Viscosimtry test: Provides the viscosifying power of the polymer
solution, allowing the calculation of polymer consumption (rheometer)
·
Adsorption: Evaluates the adsorption levels in core sample
(UV-spectrophotometric.)
·
Resistance factor: Evaluates the possibility of reduction in
permeability due to polymer injection, if any.
·
IFT measurement: Determine the optimum concentration of the SP
solution (tensiometer).
·
Thermal Stability test: evaluate the performance of SP solution
at various temperatures
Chemicals
under evaluation
·
Primarily,
the already available chemicals such as Phenyl-polyacrylamide (PPAM), Hydrolyzed
Polyacrylamide (HPAM) and KYPAM will be used in experiments.
·
Surfactants
such as Linear alcohol ethoxylates, Nonylphenol ethoxylates, petroleum
sulfonates and synthetic alkyl sulfonates, Alkyl Ether Carboxylates
Simulation modelling
A probabilistic approach will be taken
toward the findings of this project by means of embracing the uncertainties
associated with subsurface and surface elements. Through Risk and uncertainty
analysis it would be possible to reduce the error margins. This can be achieved
by means of Experimental Design in order to take into consideration all
possible development scenarios of a project.
References
§
Sheng.
J, 2013. A Comprehensive Review of Alkaline-Surfactant-Polymer (ASP) Flooding.
Paper SPE 165358, presented at the 2013 SPE Western Regional &AAPG Pacific
Section Meeting, California, USA, 19-25 April 2013.
§
Manrique,
E; Muci.V.E; Gurfinkel, M. “EOR Field Experiences in Carbonate Reservoirs in
the United States”. SPE 1000063, paper presented at the 2006 SPE/DOE Symposium
on Improved Oil Recovery held in Tulsa, Oklahoma, 22-26 April, 2006.
§
Shen,
P., Wang, J., Yuan, S., Zhon, T., Jla, X., “Study of Enhanced-Oil-Recovery
Mechanism of Alkali/Surfactant/Polymer Flooding in Porous Media from Experiments
“. SPE 126126 presented at the
International Petroleum Technology Conference held in Dubai, 4-6 December 2007.
§
Wever,
D., Broekhuis, F., Polymers for enhanced oil recovery: A paradigm for
structure-property releationship in aqueous. Pogress in Polymer Science.
36,11,p.1588-1628, November 2011.
§
Zhang,
J., Ravikiran, R., Freiberg, D., Thomas, C., 2012. ASP Formulation Design for
Heavy Oil. Paper SPE 153570, presented at the Eighteenth SPE Improved Oil
Recovery Symposium, Tulsa, Oklahoma, 14-18 April 2012.
Author.
Arash Farhadi.












0 comments: